Parents Guide to the COVID-19 Kids Vaccine
A guide for parents and caregivers on the COVID-19 vaccine for kids under 12
The Pfizer-BioNTech COVID-19 vaccine has shown to be safe and effective for kids aged 5 to 11, so why can't we start vaccinating these younger children?
Although the vaccine for 5 to 11 year olds has finished the phase 2/3 trials, we can't administer the vaccine until the FDA grants emergency use authorization or approves the vaccine. Some experts estimate that the vaccine will be authorized and available as early as November, others think closer to end of 2021 or January 2022.
Kids Vaccine Approval Process
How does the vaccine approval process work and where are different age groups at in the process?
The process for determining that a vaccine is safe and effective consists of 4 key stages:
- Phase 1. This stage tests the safety and dosage of the vaccine.
- Phase 2/3. This stage tests safety and how well the vaccine works.
- Emergency Use Authorization. This allows people to start receiving the vaccine.
- Approved. This grants full approval to the vaccine.
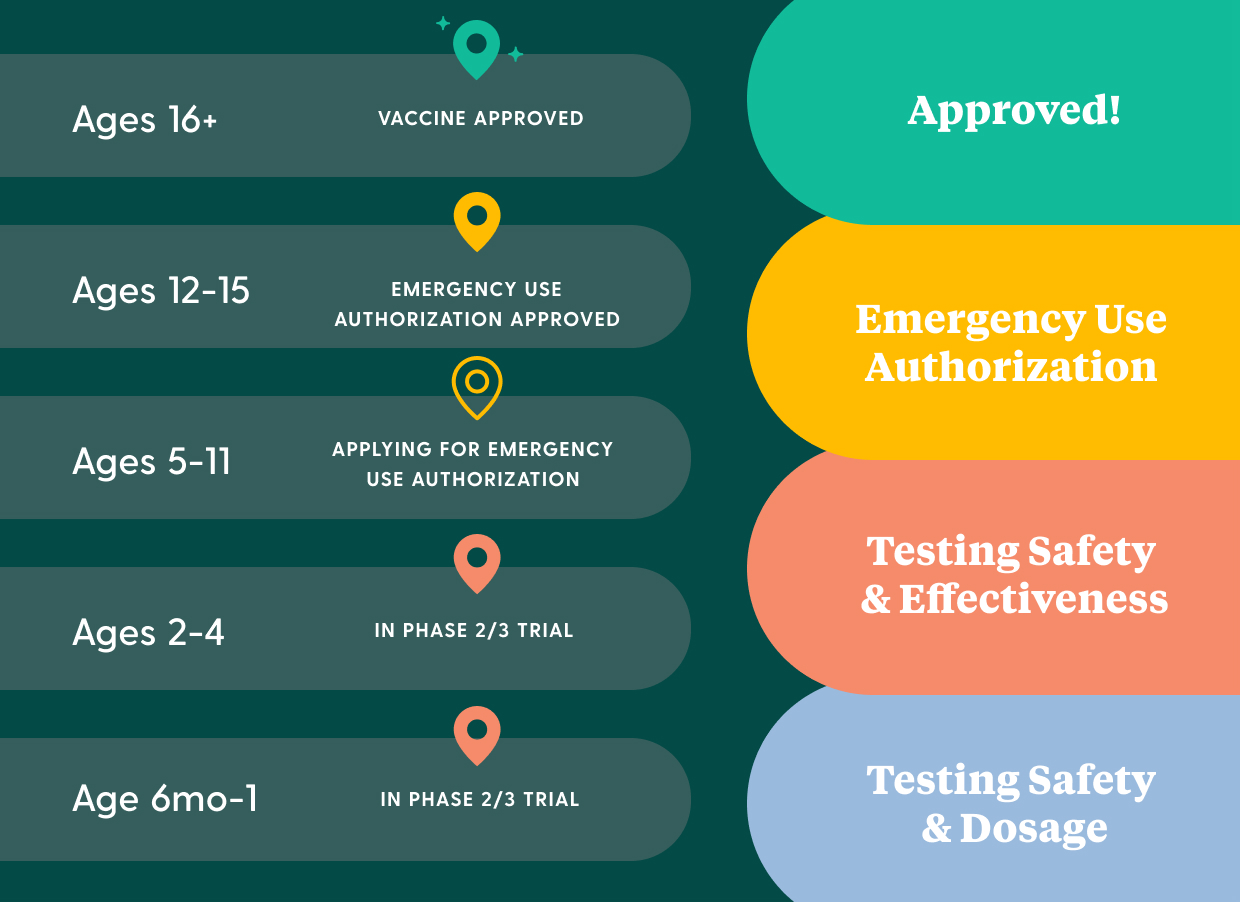
But just because the vaccine is approved for some age groups, doesn't mean that it's approved for all age groups. The COVID-19 vaccine is being tested for safety and effectiveness across 5 important age groups:
- People 16 years old and older. Vaccine has been approved.
- Kids aged 12 to 15. Vaccine has been authorized.
- Kids aged 5 to 11. Vaccine has finished phase 2/3 trials.
- Kids aged 2 to 4. Vaccine is in phase 2/3 trials. (Results will likely be available at the end of 2021)
- Kids aged 6 months to 1. Vaccine is in phase 2/3 trials. (Results will likely be available at the end of 2021)
Kids Vaccine Availability
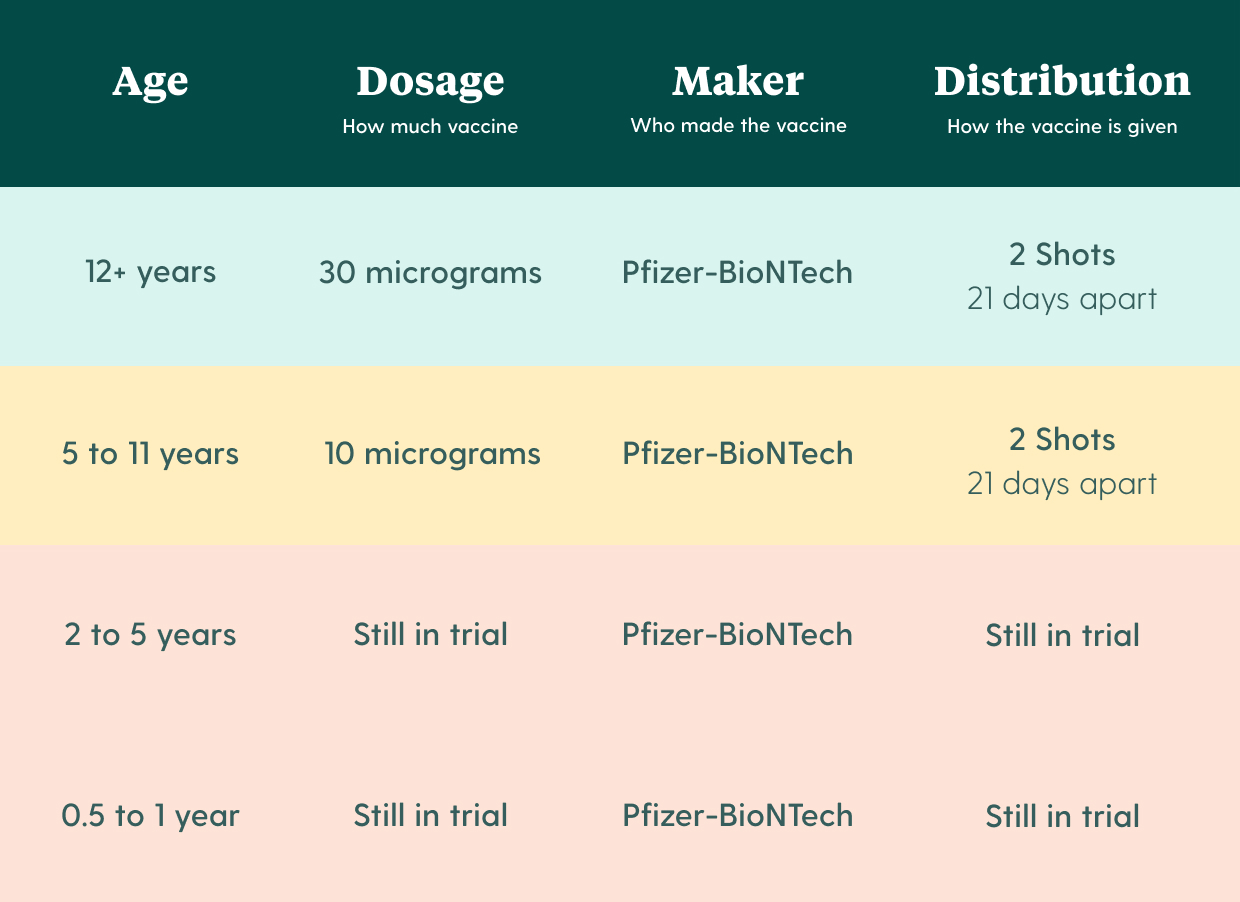
The kids vaccine and adult vaccine are the same vaccine. The only difference is the dosage amount - children aged 5 to 11 get 1/3 of the adult dose of the vaccine micrograms. However, it's unclear if we'll be able to use the adult vaccine for children.
In theory, practitioners could draw 1/3 of the adult dose to give to children, but this would require very precise measurement from practitioners. Vaccine manufacturers may decide to send a new formulation of the vaccine that is more diluted than the adult vaccine, making it easier and more precise for practitioners to measure.
Kids Vaccine Timeline
This opens up two possible timelines for vaccine administration:
- If the FDA approves using 1/3 of the adult doses, we won't have to wait for a new vaccine to be manufactured. We can start vaccinating children 5 to 11 immediately after the vaccine is authorized by the FDA.
- If the FDA approves a new children's formulation of the vaccine, we will have to wait for the new formulation to be manufactured and distributed.
We won't know how the kids vaccine will be administered (using 1/3 of the adult formulation or using a new kids formulation) until the vaccine is authorized by the FDA.
Dr. Scott Gottlieb, a former FDA commissioner and current Pfizer board member, estimates that the FDA will grant emergency authorization of the vaccine for kids aged 5 to 11 by the end of October to early November.
Dr. Francis Collins, director of the National Institutes of Health, estimates that full approval will be granted near the end of 2021.
Kids Vaccine vs Adult Vaccine
Kids Vaccine Dosage
The 5 to 11 year old vaccine is a two-dose regimen that is administered 21 days apart. It uses a 10 microgram dose instead of the 30 microgram dose used for people over 12 years old.
Kids Vaccine Side Effects
The side effects for 5 to 11 year olds were comparable to the side effects of people aged 16 to 25 years old.
Kids Vaccine Risk Levels
Just as in adults, children with chronic medical problems, co-morbid conditions, or who are immunocompromised would significantly benefit from Covid 19 vaccination. Healthy children would also benefit due to the risk of MISC, and would mean >28 million people in the US would be eligible for the vaccine.
Kids Vaccine Updates
- Sept 20, 2021 - The Pfizer-BioNTech COVID-19 vaccine finished phase 2/3 trials for kids age 5 to 11. It was found to be safe and effective in smaller dosages.
- Aug 23, 2021 - The FDA approved the Pfizer-BioNTech COVID-19 vaccine for people people 16 years of age and older.
- May 10, 2021 - The FDA granted emergency authorization use of the Pfizer-BioNTech COVID-19 vaccine to kids aged 12 to 15.
- Dec 11, 2020 - The FDA granted emergency authorization use of the Pfizer-BioNTech COVID-19 vaccine to people 16 years of age and older.



